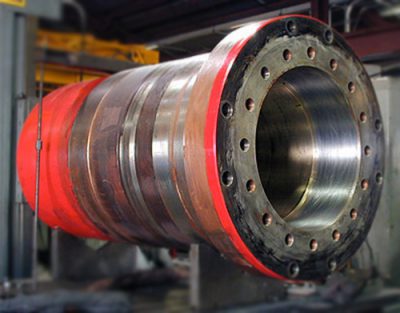
Project Description
HARD CHROME PLATING
Hard chrome plating is an electroplating process in which chromium is deposited from a chromic acid solution. Thickness of hard chrome plating ranges from 25 to 500µm. Various types of hard chrome include micro-cracked chromium, micro-porous chromium, porous chromium and crack free chromium. It is essential that the micro-cracked and porous coatings have a minimum thickness between 80-120µm in order to confer adequate corrosion resistance. Micro-cracked chromium has a Vickers hardness of 800-1000 kg/mm2, while crack-free chromium has Vickers hardness between 425-700 kg/mm2. The formation of micro-porous chromium is achieved by a specialised plating method involving the use of inert suspended particles. Porous chrome plating is developed by etching electrodeposited chromium. These are designed to retain lubricant, for sliding and bearing type applications.
In the surface engineering and finishing industry there are many different processes and coatings which are applied to a substrate to change its surface properties. Amongst the many surface coatings in use across a wide range of industries to improve the substrates performances is hard chrome plating. Here we take a brief look at this plating process.
What is hard chrome plating?
Hard chrome plating is an electrolytic process in which chromium is deposited onto a metal substrate giving it enhanced properties for durability, wear and hardness. The electrolytic process is generally achieved by passing an electric current through a chromic acid solution (called an electrolyte bath) between two electrodes, one of which will be the substrate which is to be plated. When the current flows between the electrodes, a chemical reaction is induced whereby the chromium metal from the solution is deposited in a thin layer on the component to be plated.
What is hard chrome plating used for?
This is a very effective treatment for a variety of metals/alloys including steel, copper and brass which are used in many modern technical applications. It is commonly used in situations where the component needs to be resistant to corrosion such as hydraulic piston rods, gear shafts, and motorcycle forks used in the automotive sector. It is also ideal for processes that require low-friction surfaces for delicate products like textiles and paper.
Hard chrome plating has a number of beneficial properties which include:
- Low-friction
- Abrasion resistance
- Hardness
- Excellent wear and corrosion resistance
Unfortunately, due to the toxicity of substances used in the production of the coating, hard chrome plating is controlled by a number of regulations in respect of the use and disposal of the chromium substances used in the electrolytic process.
Metal Finishing
Discovering the world of metal finishing
In the metal finishing industry there are many different types of processes which give a range of enhanced properties to components. Companies involved in metal finishing may specialise in just one of these processes, or may be equipped to carry out most, or all.
Here we take a look at metal finishing, what the different processes are, and the regulations which govern them.
What is metal finishing?
The main purpose of metal finishing is to give enhanced properties to various substrates (the metal that is to be coated or finished). It can also be used to give decorative coatings to metals.
Metals used in engineering and manufacturing, often do not have the necessary strength, durability or resistance to outside factors needed for its application. To enable the metal to be used, a coating or finish is applied so that the metal’s properties are altered to give a better performance.
The finishing processes are used to give the following improvements:
- Improved appearance
- Improved adhesion
- Greater solder ability
- Resistance to corrosion, chemicals, wear, extreme temperatures and tarnish
- Hardness
- Durability
- Electrical conductivity
- Removing of surface flaws and burrs
- Control of surface friction
The many uses of metal finishing
Metal finishing and surface coating techniques are used for many applications, from new technologies and processes to traditional manufacturing, where the surface of the metal is subjected to a number of harsh processes.
Sectors as wide ranging as automotive, aerospace, defence, power supply, electronics, petrochemical, medical, chemical and engineering, to name but a few, rely on products which have been subjected to the finishing process.
Types of metal finishes
There are several different ways to carry out metal finishing, some more commonly used than others. These include, but are not limited to:
Electroplating – This process involves passing an electrical current between two electrodes (one, being the substrate that is to have the metal finish) through an electrolyte solution. The electrical current causes a chemical reaction that results in the atoms of the electrolyte being split up and deposited on the electrode. Therefore, if you wish to coat a substrate with silver, then the electrolyte would need to be made up of a silver-based solution. Electroplating can be carried out on a wide range of metals including, gold, silver, tin, copper, cadmium, nickel, platinum, zinc and lead
Anodising – The process of anodising is much the same as electroplating, whereby an oxide film is formed on the substrate by passing an electrical current through an electrolytic solution. Aluminium is commonly used within anodising, while both magnesium and titanium can also be anodised.
Electroless plating – this process involves applying a uniform layer(s) of a metal to a substrate without the need for an electrical charge. Nickel is often used for this process as it gives a uniform thickness on the substrate even on awkward shaped objects.
Passivation – This process is used to improve the surface condition of stainless steel by dissolving any iron that has been deposited during manufacturing. This is achieved by placing the stainless steel into an acid solution to dissolve the residual iron and to form a thin oxide layer on the surface
What is Industrial Hard Chrome?
The electrolytic deposition of chromium to the surfaces of other materials, primarily metals, occurs when electrical energy supplied to electrodes in a solution consisting primarily of chromic acid is converted to chemical energy to produce chromium metal.
Frequently, when expensive machined parts are damaged or worn and no longer functional, hard chrome plating can be used to build up the lost metal and the parts can then be ground to their original tolerances. In many cases the wear life will have improved.
On new parts hard chrome is used to improve the durability of a variety of components of industrial equipment. The printing industry makes use of copper engraved plates and cylinders which are hard chrome plated for corrosion and wear resistance. Oil exploration equipment and production machinery of all types have many of their component parts chrome plated to extend their in-service life and to reduce costly downtime. Hydraulic equipment utilizes chrome plated shafting to extend service life in corrosive industrial environments.
Unique Combination of Properties
The success of hard chrome plate in Industrial applications may probably be attributed to its unique combination of properties not possessed by any other one material available commercially. The most important of these are hardness, adhesion, wear resistance, non-wetting qualities, and low coefficient of friction. In many instances all these properties are important for successful commercial applications.
The hardness alone would not be sufficient to secure widespread use, because a number of other hard materials, or hardening processes are available. It is the combination of very great hardness with extremely good corrosion resistance, and very low coefficient of friction or unique surface qualities, which has given such remarkable results ln many applications of chromium plate. To this should be added the ease of stripping and replating for repeated salvage in cases where the plating wears beyond permissible limits.
Properties and Benefits of Industrial Hard Chrome Plating
- Wear & abrasion resistance
- Lubricity
- Hardness
- Durability
- Adhesion & bonding
- Low coefficient of friction in metal parts
- Prevents seizing & galling
- Restores the dimensions of undersized parts
- Extends equipment in-service life, reducing costly downtime
Military Specification
Military Specification QQC-320 governs the use of both hard chrome and decorative chrome in military and aircraft applications.
Materials Suitable for Chrome Plating
Any ferrous and most non-ferrous metals are suitable for chrome plating. Exceptions are magnesium and titanium, which usually require an under-layer of zinc, copper or nickel as well as special plating techniques. Aluminum can be successfully chrome plated, but usually requires a copper or nickel under-layer, although some alloys have been plated without an undercoat. In recent years, new techniques have permitted the application of decorative chrome on plastics.